This set of Engineering Materials Science "Numerical Problems Questions & Answers" are based on "PHASE DIAGRAM"
PHASE DIAGRAM
NUMERICAL PROBLEMS
Hello reader, here you can find some Numerical Problems with answers. But before reading this I will suggest you take a practice test on these questions given below. so you can understand your strength on this topic.
Click here to go Practice Test section on this topic namely Phase Diagram.
But if you want to read this topic first and then practice it then read it carefully. The Numerical Problems based on the Phase Diagram are given below:
PART - I
1) What are the total numbers of variables of a system of two components when the number of phases is 3?
Correct Answer is "D"
Total number of variable = P (C-1) + 2 = 3 (2–1) + 2 =5
2) At atmospheric pressure (pressure arbitrarily is chosen), a material of unknown composition shows 3 phases in equilibrium at 987 K. What is the minimum number of components in the system?
Correct Answer is "C"
For minmum number of components, F = 0 = C – P + 1(since pressure constant)
Or C = P – 1 = 3 – 1 = 2
Or C = P – 1 = 3 – 1 = 2
3) What are the degrees of freedom of a system of two components when the number of phases is one, two, three respectively?
Correct Answer is "A"
D.O.F for 2 components 1 phase system = 2 – 1 + 2 = 3
D.O.F for 2 components 2 phase system = 2 – 2 + 2 = 2
D.O.F for 2 components 3 phase system = 2 –3 + 2 = 1
D.O.F for 2 components 2 phase system = 2 – 2 + 2 = 2
D.O.F for 2 components 3 phase system = 2 –3 + 2 = 1
4) The H2O–NaCl system has the following eutectic reaction occurring at –21°C:
Liquid (23.3% NaCl)↔ice(0% NaCl)+ Salt (100% NaCl) (forward direction→cooling)
How much pure water can be extracted from seawater (3.5% NaCl) by cooling to –20.9°C?
Correct Answer is "C"
For the given eutectic reaction utilizing LEVER rule, extracted pure water = {(23.3-3.5)/(23.3-0)} = 0.849
5) A Eutectoid steel is slowly cooled from a temperature of 850°C to a temperature just below 727°C. The percentage of ferrite and cementite are respectively
 |
| Image may be subjected to copyright |
Correct Answer is "D"
Utilizing LEVER rule at eutectoid isotherm,
Amount of ferrite = {(6.7-0.76)/(6.7-0.022)} × 100% = 88.9%
Amount of cementite = {(0.76-0.022)/(6.7-0.022)} × 100% = 11.1%
Amount of ferrite = {(6.7-0.76)/(6.7-0.022)} × 100% = 88.9%
Amount of cementite = {(0.76-0.022)/(6.7-0.022)} × 100% = 11.1%
6) In the Pb–Sn system, the fraction of α phase in an alloy of 40% Sn at 184°C and 182°C are respectively.
 |
| Image may be subjected to copyright |
Correct Answer is "C"
Utilizing the LEVER rule above and below the eutectic isotherm,
Fraction of α phase at 184°C = {(61.9-40)/(61.9-18.3)} = 0.50
Fraction of α phase at 182°C = {(97.8-40)/(97.8-18.3)} = 0.73
Fraction of α phase at 184°C = {(61.9-40)/(61.9-18.3)} = 0.50
Fraction of α phase at 182°C = {(97.8-40)/(97.8-18.3)} = 0.73
7) What is the fraction of pro eutectoid cementite in 1.7% steel?
 |
| Image may be subjected to copyright |
Correct Answer is "B"
Utilizing LEVER rule, Fraction of proeutectoid cementite = {(1.7-0.76)/(6.7-0.76)} = 0.158
8) In the Pb–Sn system, calculate the alloy composition at which the fraction of total α is 4 times the fraction of the β phase at 182°C.
 |
| Image may be subjected to copyright |
Correct Answer is "A"
Let the alloy composition be x% Sn
Hence, = {(97.8-x)/(97.8-18.3)} = 4 × = {(x-18.3)/(97.8-18.3)}
Or, 5x = 171
Or, x = 34.2
Hence, = {(97.8-x)/(97.8-18.3)} = 4 × = {(x-18.3)/(97.8-18.3)}
Or, 5x = 171
Or, x = 34.2
9) A 70 wt% Ni–30 wt% Cu alloy is slowly cooled from 1400°C to 1200°C. What is the composition of this last remaining liquid phase?
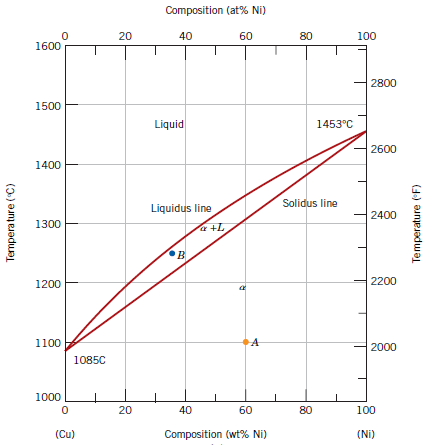 |
| Image may be subjected to copyright |
Correct Answer is "B"
If we draw an upward vertical from 70% Ni it cuts the solidus line at some point.
Then we draw a horizontal tie line from that cutting point which cuts the liquidus line at some point (left-side).
From the intersection of tie line and liquidus line, we draw a downward vertical which touches the composition axis at approximately 57% Ni.
Since it is a binary phase diagram 57% Ni = 43% Cu.
Then we draw a horizontal tie line from that cutting point which cuts the liquidus line at some point (left-side).
From the intersection of tie line and liquidus line, we draw a downward vertical which touches the composition axis at approximately 57% Ni.
Since it is a binary phase diagram 57% Ni = 43% Cu.
10) If the fraction of liquid with 24% B, which is in equilibrium with solid of 67% B, is 0.4, the overall composition is
Correct Answer is "A"
Let the overall composition be x% B
From LEVER rule, {(67-x)/(67-24)} = 0.4 = fraction of liquid
Or, x = 49.8
From LEVER rule, {(67-x)/(67-24)} = 0.4 = fraction of liquid
Or, x = 49.8
Do you read all the questions and answers given above carefully? let's make a practice on all the questions given above.
Go to: Practice Test on Phase Diagram (Numerical Problems) PART - I
Suggested Topics:
Disclaimer:
Some of the questions given above may be similar to questions from some books and other online available sources but the answers to all the questions are genuinely made by admin@materialscienceonline.
Let me know if this post is helpful or not?
I am trying my best to gather more and more objective type questions on this site for your best practice. If you have any queries about any questions given above then don't hesitate to comment below or you can also directly contact me through materialscienceonline4u@gmail.com.
MaterialScienceOnline is made to provide you all types of chapter-wise objective (Multiple Choice, Multiple Select and Numerical) questions, answers, and explanations as well as a chapter-wise various practice tests or quiz to catalyst your competitive exam preparation. Various top-rated Material Science and Engineering pdf books are also available here.
Thank you for visiting this site. If you think this post is helpful then share this post as well as this website with your friends.







No comments:
Post a Comment
Let me know about this post..