In this place, you will get all types of numerical problem-solving formulas on the topic of "PHASE DIAGRAM" on one page.
This formula will help you to solve different types of problems on "PHASE DIAGRAM" very easily.
Check out all this formula given below before solving Numerical Problems on "PHASE DIAGRAM" given on this site or anywhere.
PHASE DIAGRAM
FORMULA
Formula 1
Used to Calculate: Mass fraction of liquid phase, binary isomorphous system
WL = {(Cα - C0) / (Cα - CL)}
Where,
WL = Mass fraction of liquid phase, binary isomorphous system
Cα = Composition of α phase
C0 = Overall alloy composition (in terms of one of the components)
CL = Composition of L (liquid) phase
WL = Mass fraction of liquid phase, binary isomorphous system
Cα = Composition of α phase
C0 = Overall alloy composition (in terms of one of the components)
CL = Composition of L (liquid) phase
Formula 2
Used to Calculate: Mass fraction of α solid-solution phase, binary isomorphous system
Wα = {(C0 - CL) / (Cα - CL)}
Where,
Wα = Mass fraction of α solid-solution phase, binary isomorphous system
C0 = Overall alloy composition (in terms of one of the components)
Cα = Composition of α phase
CL = Composition of L (liquid) phase
Wα = Mass fraction of α solid-solution phase, binary isomorphous system
C0 = Overall alloy composition (in terms of one of the components)
Cα = Composition of α phase
CL = Composition of L (liquid) phase
Formula 3
Used to Calculate: Volume fraction of α phase
Vα = {vα / (vα + vβ)}
Where,
Vα = Volume fraction of α phase
vα = Volum of α phase
vβ = Volum of β phase
Vα = Volume fraction of α phase
vα = Volum of α phase
vβ = Volum of β phase
Formula 4
Used to Calculate: Volume Fraction of α phase from the mass fraction
Vα = [(Wα/ρα) / { (Wα/ρα) + (Wβ/ρβ)}]
Where,
Vα = Volume fraction of α phase
Wα = Mass fraction of α solid-solution phase
ρα = Density of the α phase
Wβ = Mass fraction of β solid-solution phase
ρβ = Density of β phase
Vα = Volume fraction of α phase
Wα = Mass fraction of α solid-solution phase
ρα = Density of the α phase
Wβ = Mass fraction of β solid-solution phase
ρβ = Density of β phase
Formula 5
Used to Calculate: Mass fraction of α phase from volume fraction
Wα = [(Vαρα) / {(Vαρα) + (Vβρβ)}]
Where,
Wα = Mass fraction of α solid-solution phase
Vα = Volume fraction of α phase
ρα = Density of the α phase
Vβ = Volume fraction of β phase
ρβ = Density of β phase
Wα = Mass fraction of α solid-solution phase
Vα = Volume fraction of α phase
ρα = Density of the α phase
Vβ = Volume fraction of β phase
ρβ = Density of β phase
Formula 6
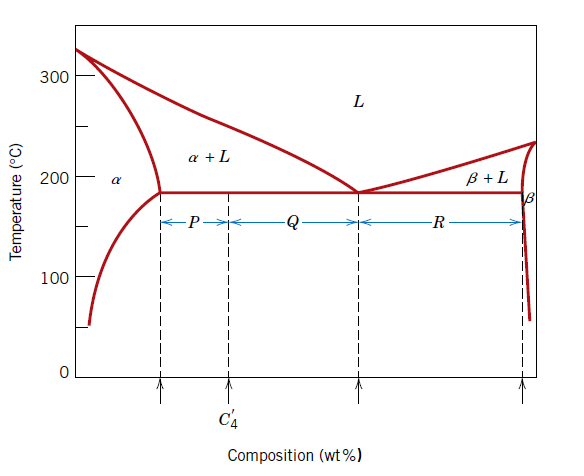
Used to Calculate: Mass fraction of eutectic micro constituent for binary eutectic system
We = P/(P + Q)
Where,
We = Mass fraction of eutectic micro constituent for binary eutectic system
P, Q = Lengths of tie-line segments as shown in figure
We = Mass fraction of eutectic micro constituent for binary eutectic system
P, Q = Lengths of tie-line segments as shown in figure
Formula 7
Used to Calculate: Mass fraction of primary α micro constituent for binary eutectic system
Wα' = Q/(P + Q)
Where,
Wα' = Mass fraction of primary α micro constituent for binary eutectic system
P, Q = Lengths of tie-line segments as shown in figure
Wα' = Mass fraction of primary α micro constituent for binary eutectic system
P, Q = Lengths of tie-line segments as shown in figure
Formula 8
Used to Calculate: Mass fraction of total α phase for a binary eutectic system
Wα = (Q + R)/(P + Q + R)
Where,
Wα = Mass fraction of total α phase for a binary eutectic system
P, Q, R = Lengths of tie-line segments as shown in figure
Wα = Mass fraction of total α phase for a binary eutectic system
P, Q, R = Lengths of tie-line segments as shown in figure
Formula 9
Used to Calculate: Mass fraction of β phase for a binary eutectic system
Wβ = P/(P + Q + R)
Where,
Wβ = Mass fraction of β phase for a binary eutectic system
P, Q, R = Lengths of tie-line segments as shown in figure
Wβ = Mass fraction of β phase for a binary eutectic system
P, Q, R = Lengths of tie-line segments as shown in figure
Formula 10
Used to Calculate: Number of Phase or Component present in a system, Number of degrees of freedom (Gibbs phase rule)
P + F = C + N
Where,
P = Number of phase present in a given system
F = Number of Degrees of freedom or Number of externally controlled variables (e.g., temperature, pressure, composition) that must be specified to define the state of the system completely.
C = Number of components in the system
N = Number of noncompositional variables (e.g., pressure and temperature)
P = Number of phase present in a given system
F = Number of Degrees of freedom or Number of externally controlled variables (e.g., temperature, pressure, composition) that must be specified to define the state of the system completely.
C = Number of components in the system
N = Number of noncompositional variables (e.g., pressure and temperature)
Formula 11
Used to Calculate: Total Number of Variables in a system
V = P(C - 1) + N
Where,
V = Total number of variables in a system
P = Number of phase present in a given system
C = Number of components in the system
N = Number of noncompositional variables (e.g., pressure and temperature)
P(C - 1) = Total number of composition variables
V = Total number of variables in a system
P = Number of phase present in a given system
C = Number of components in the system
N = Number of noncompositional variables (e.g., pressure and temperature)
P(C - 1) = Total number of composition variables
Formula 12
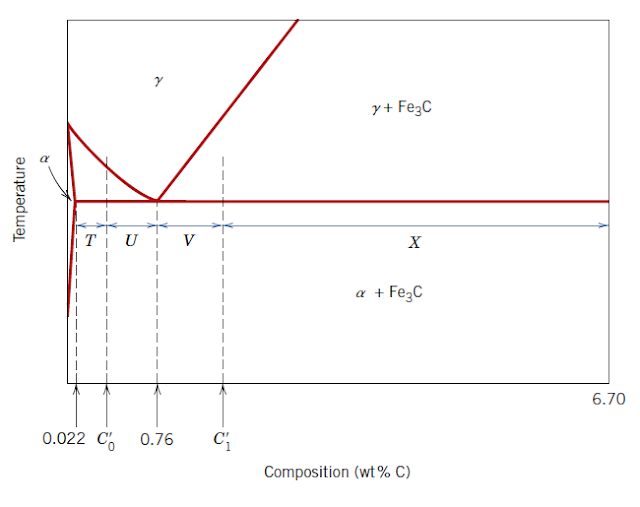
Used to Calculate: Mass fraction of pearlite for a hypoeutectoid Fe - C alloy
WP = T/(T + U) = {(C0' - 0.022) / 0.74}
Where,
WP = Mass fraction of pearlite
C0' = Composition of a hypoeutectoid alloy in weight percent carbon
T, U = Lengths of tie-line segments as shown in figure
WP = Mass fraction of pearlite
C0' = Composition of a hypoeutectoid alloy in weight percent carbon
T, U = Lengths of tie-line segments as shown in figure
Formula 13
Used to Calculate: Mass fraction of pro eutectoid α ferrite phase for a hypoeutectoid Fe - C alloy
Wα' = U/(T + U) = {(0.76 - C0') / 0.74}
Where,
Wα' = Mass fraction of proeutectoid α ferrite phase
C0' = Composition of a hypoeutectoid alloy in weight percent carbon
T, U = Lengths of tie-line segments as shown in figure
Wα' = Mass fraction of proeutectoid α ferrite phase
C0' = Composition of a hypoeutectoid alloy in weight percent carbon
T, U = Lengths of tie-line segments as shown in figure
Formula 14
Used to Calculate: Mass fraction of pearlite for a hypereutectoid Fe - C alloy
WP = X/(V + X) = {(6.70 - C1') / 5.94}
Where,
WP = Mass fraction of pearlite
C1' = Composition of a hypereutectoid alloy in weight percent carbon
X, V = Lengths of tie-line segments as shown in figure
WP = Mass fraction of pearlite
C1' = Composition of a hypereutectoid alloy in weight percent carbon
X, V = Lengths of tie-line segments as shown in figure
Formula 15
Used to Calculate: Mass fraction of pro eutectoid Fe3C phase for a hypoeutectoid Fe - C alloy
W(Fe3C) = V/(V + X) = {(C1' - 0.76) / 5.94}
Where,
W(Fe3C) = Mass fraction of proeutectoid Fe3C phase
C1' = Composition of a hypereutectoid alloy in weight percent carbon
X, V = Lengths of tie-line segments as shown in figure
W(Fe3C) = Mass fraction of proeutectoid Fe3C phase
C1' = Composition of a hypereutectoid alloy in weight percent carbon
X, V = Lengths of tie-line segments as shown in figure
REFERENCES:
Materials Science and Engineering: an Introduction (9E) by William D. Callister, Jr., and David G. Rethwisch.
Click here to know details about this book and download it in pdf format.
Materials Science and Engineering: A First Course by Raghavan.
Click here to know details about this book and download it in pdf format.
Suggestions to Readers:
Hey Readers, Do you read carefully all the formulas based on "PHASE DIAGRAM" given above? so why are you waiting for? Let's see how these formulas are used in Numerical problems.
Click here to visit the Numerical Problems on "PHASE DIAGRAM".
Or you can also test your skill by practice some problems based on "PHASE DIAGRAM".
Click here to practice Numerical problems on "PHASE DIAGRAM".
MaterialScienceOnline is made to provide you all types of chapter-wise objective (Multiple Choice, Multiple Select and Numerical) questions, answers, and explanations as well as a chapter-wise various practice tests or quiz to catalyst your competitive exam preparation. Various top-rated Material Science and Engineering pdf books are also available here.
Thank you for visiting this site. If you think this post is helpful then share this post as well as this website with your friends.









No comments:
Post a Comment
Let me know about this post..